China has been promoting the construction of medical device traceability system in recent years and made steady and solid progress. In July 2019, Pilot Work Plan for Unique Identification System of Medical device jointly printed by the Comprehensive Division of National Medical Products Administration and the General Office of National Health Commission of the People’s Republic of China was officially released, which marks that the unique identification system pilot work of medical device is formally started in China. While the medical device traceability system construction enters the new era, enterprises in the industry also accelerate the construction of medical device traceability system together with the supervision progress.
The whole process of medical device can be traced, and the whole-process supervision era is coming
With the constant development of medical technology, the variety and type of medical high-value consumables are also increased rapidly. In the past, many links in hospitals were generally carried out artificially, codes such as product registration and filing number, centralized procurement code, information system code of medical institution and medical insurance charging code often coexisted, they have own system and “isolated” from each other. Therefore, serious asymmetric information and non-transparent traces often occur, which is unfavorable for the supervision and governance of medical devices.
The establishment of medical device traceability system means that the whole process from production, processing, circulation, distribution and use can be traced and supervised, and it is an important method to ensure the integrity and safety of medical device supply chain. UDI is recognized as an advanced way for supervising the international medical device as well as the international language of medical devices. The formal and unified implementation of unique equipment identification in China marks the breakthrough of the last restriction and the realization of unified supervision of medical devices in domestic and abroad. The unified traceability system which takes “unique code for device and the unique device identification (UDI) as the means is equivalent to the only “ID card” of medical device! 
Breaking through the supply chain and realizing five-point linkage
Since China has accelerated the construction of medical device traceability system, Acctrue Technology has established the “medical device traceability cloud platform”, many medical device manufacturers such as BioRegen Biomedical (Changzhou) Co., Ltd., Jinling Pharmaceutical Factory of JINLING PHARMACEUTICAL CO., LTD. has completed data docking with the platform, realized visual management of supply chain and assisted the medical industry for healthy development based on big data. The “Medical device traceability cloud platform” of Acctrue Technology can realize five-point linkage, that is, connecting with the production enterprises, circulation enterprises, national static database, hospitals and patients, breaking through the supply chain and realizing closed-loop management. Docking with the national static database help medical device enterprises dock with the government platform, assist the food and drug supervision management department to call and manage data according to the regulation demand and provide more guarantee for consumers.
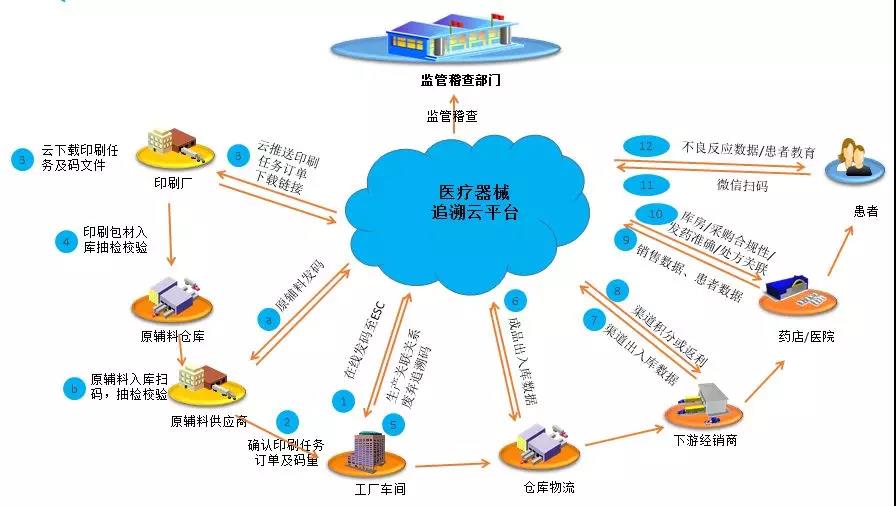
1、Professional medical device traceability cloud platform
The “medical device traceability platform” takes “unique code for product” and internet cloud as the basis and provides one-stop traceability service for enterprises such as professional consultation, customized solution, site implementation, operation and maintenance and big data application based on internet big data and cloud computing.
2、“Cloud + terminal” fully covering the medical device traceability platform
The “medical device traceability platform” integrates traceability supervision, supply chain control and terminal management into a whole, assists enterprises for the quick implementation of “cloud + terminal” management from equipment layer, production line layer, factory layer, enterprise layer and market layer, automatically records, marks and analyzes the production and operation activity data, helps enterprises to observe the whole supply chain network in real time, realizes whole process visual tracing from production, circulation distribution, logistics transportation and terminal sales, assists enterprises to quickly establish the medical device traceability system that meets the national and market supervision requirements, constructs the ecology of information service big data and facilitates interaction between the hospitals and patients and the enterprises.
3、Medical device traceability platform with high compatibility, compliance and safety
Firstly, the “medical device traceability platform” can realize seamless docking of the original management system of enterprises (such as ERP, MES and WMS). Secondly, Acctrue Technology deeply understands the industrial policies and standards of global medical devices, has various codes such as UDI and GS1 and assists enterprises to create unique identification, tagging and upload/download data. Finally, the “data safety guarantee system” of Acctrue Technology guarantees the safe and stable operation of data from multiple dimensions such as unified data standard, management, verification and synergy efficiency. The whole data can be collected, generated, stored, transmitted and printed in a closed environment without leakage, tampering and duplication.
4、Pure third-party medical device traceability platform
Accture Technology is a pure system service provider for guaranteeing the business data safety, it is never involved in manufacturing or sales of medical devices but always adheres to the cooperation principle of sincerity and trust.
Beijing Acctrue Technology Co., Ltd. focuses on the whole industry chain traceability of medicine and medical care, deeply ploughing the pharmaceutical industry for dozens of years, provides services for more than 5,000 domestic enterprises and 50 foreign enterprises, owns over 15,000 production lines and has strong solution capacity and rich experience in production line. Acctrue has been committed to promoting the application of GS1 unified code in medical industry for a long time, breaks through all links of medical devices such as production, operation, circulation and use, realizes information interconnection and completes the whole industry chain tracing from production source to the final clinical application.


