1. Consumption upgrading drives the demand growth of imported pharmaceutical products
Due to the structural change in Chinese resident consumption upgrading caused by factors including rising income, aging population and technological progress, the Chinese consumers’ demand for special overseas consumer goods such as high-quality drugs and medical apparatus and instruments has been on the rise year after year, and their strong consumption power plays a more and more important role in the world. The data show that China's pharmaceutical imports from 2015 to 2019 increased from 106064 tons to 158022 tons, and the amount of pharmaceutical imports by 2019 exceeded 246,233,750,000 yuan.
2. Consumption demand promotes the reform of pharmaceutical supply and marketing mode
Although the consumers have a great demand for imported pharmaceutical products, the previous cross-border consumption market of drugs and medical apparatus and instruments survives mainly by means of informal channels such as cross-border online shopping, purchasing agents, etc. On December 30, 2019, Beijing Medical Products Administration in company with Beijing Municipal Bureau of Commerce, Beijing Customs and Beijing Tianzhu Free Trade Zone Management Committee issued the “Beijing Cross-border E-commerce Pharmaceutical Product Sales Pilot Work Implementation Program” (hereinafter referred to as "Pilot Program"). It adopts the “Beijing model” to start the first ice-breaking trip of China's cross-border e-commerce policy in the field of pharmaceutical products, and also brings new development opportunities for the pharmaceutical industry.
3. How to guarantee the safety of drugs in cross-border circulation? Whole industry chain traceability is the key!
In order to ensure the drug safety for patients, the Pilot Program clearly specifies that the pilot enterprises have the traceability obligation, and that the cross-border pharmaceutical product traceability system shall be established in the basic principle of "one item one code, traceability of both item and code", so as to fulfill the main role of the enterprises, and to realize traceability and verification of minimum packaging unit of the cross-border pharmaceutical products inside China. It also encourages the pilot enterprises to integrate the overseas production and circulation of cross-border pharmaceutical products into the traceability system, and to implement the system of compensation in advance.
How should the pharmaceutical enterprises establish the cross-border pharmaceutical product traceability system?
Acctrue sums up the six key requirements for establishment of the imported drug whole industry chain traceability system:
| Comply with Chinese supervision laws and regulations |
| How to adopt code rules |
| How to dock with production data |
| Pay attention to the time nodes of release and implementation of new regulations by China Food and Drug Administration; Focus on how to dock with national supervision platform; |
| Meet the code rules of Chinese drug traceability code; The outer packaging has adopted the code rules of ISO/ICE (including GS1), and whether it meets the new regulation requirements of China Food and Drug Administration; |
| How to dock with the code data of the overseas production enterprises, and how to dock with the relational data and outbound data;
|
| Connect hospitals and pharmacies |
| Keep up-to-date with drug sales |
| Patient medication feedback |
| How to establish a whole chain traceability system architecture and traceability service platform in China after docking with the production data; |
| Expect to obtain the inventory and sales status of the third-party logistics, warehouse branches and channels, i.e., the response data query summary requirements; |
| Manufacturers and distributors expect to obtain the feedback from patients on rare medicines, patented special medicines and newly marketed medicines |
Among them, how to dock with the national collaboration/supervision platform and how to meet the latest industry supervision requirements are the common concerns of the relevant pharmaceutical enterprises at present.
Meanwhile, with the awareness of traceability data value in the pharmaceutical industry, more and more enterprises realize the importance of pharmaceutical data capitalization. There is a growing demand for self-management and application of data assets. A consensus appears to be emerging in all pharmaceutical enterprises, that is to build the pharmaceutical big data operation platform and to construct the new business form of highly efficient and interactive “online + offline” pharmaceutical smart retail.
How does Acctrue solve the traceability problems of the imported drug whole industry chain?
I. Three modes to support the collection/docking of imported drug serialization data
Three different data collection/docking modes are provided according to the drug coding rules in overseas production. Each mode can perfectly dock with the domestic supply chain traceability data.
Mode A:the whole serialization process is completed overseas

Mode B:the foreign manufacturers only complete the packaging material coding at all levels

Mode C:the foreign manufacturers do not give code

II. Adopt the international standard GS1 Data Matrix two-dimensional identification code, and meet the new regulation requirements of China Food and Drug Administration;
GS1 code is the unique commodity ID card worldwide, and also the participant identify label universally available. When applied in the pharmaceutical industry, it is not only a drug code, but also contains five meanings as follows:
 Ø A global system
Ø A global system
Ø A global standard
Ø A global solution
Ø World-class standardization organization (supply chain management/business field)
Ø Unified business operation under global open standard/system
II. Dock with national collaboration platform, and meet the latest industry supervision requirements
The enterprises upload the collected/docked drug traceability data to the Acctrue LinkLink pharmaceutical traceability cloud platform, and then the LinkLink platform will dock with the national collaboration platform, so as to fully meet the latest industry supervision requirements.
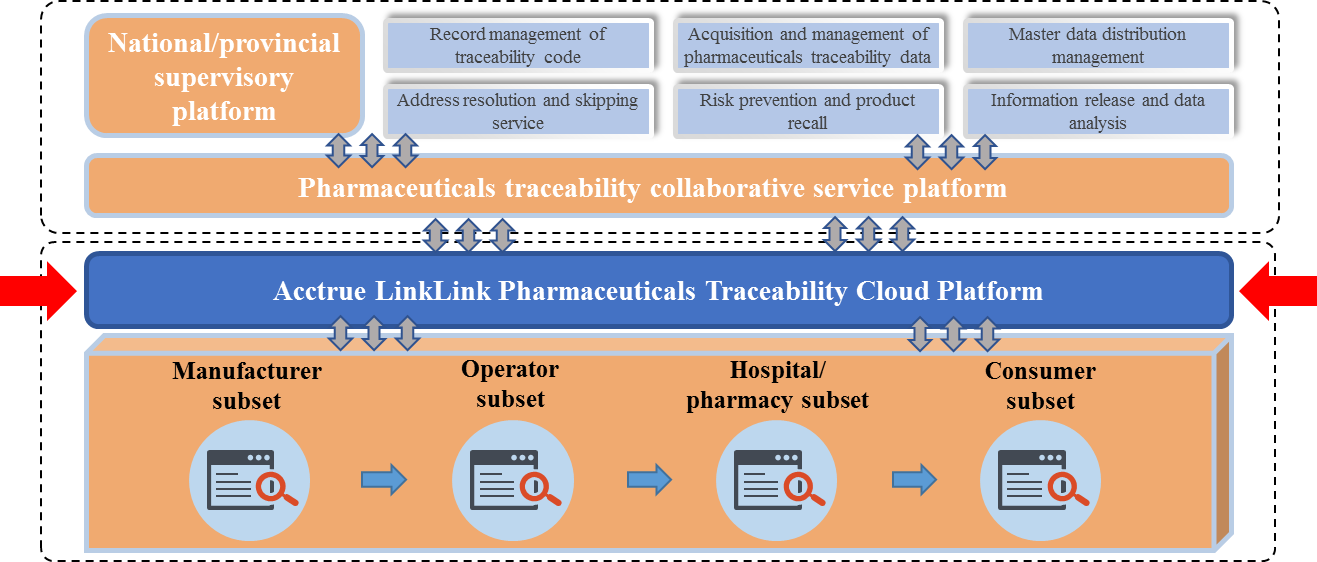
IV. Provide "Cloud + Terminal" one-stop service, and establish the imported drug GS1 whole supply chain traceability system
Acctrue provides the “Cloud + Terminal” one-stop service for pharmaceutical enterprises, adopts GS1 code as the carrier to connect all nodes from foreign manufactures to patients, establishes the whole supply chain traceability system, and enhances the imported drug whole life cycle management level in an all-round way. Meanwhile, Acctrue helps the pharmaceutical enterprises to keep up-to-date with the all-channel drug inventory and sales data in real time, and improves the industry operation efficiency.
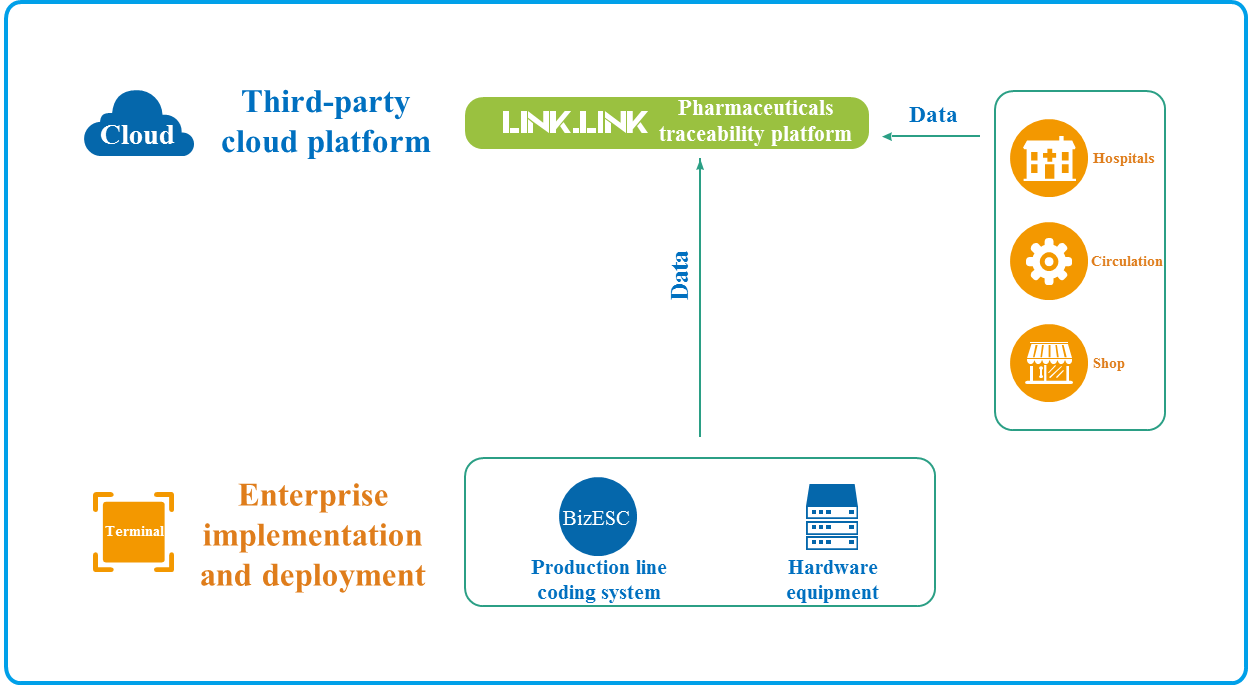
V. Construct the new business form of, and realize the application ofpharmaceutical smart retailpharmaceutical traceability data capitalization
1. Use the enterprise WeChat official account, applet and other data applications to help connect the drugs, patients and pharmacies by multiple means, and build the operation links with full-time full-domain contact for patients.
2、Build the digital pharmacies, realize the sales promotion transferred from offline to online, enhance the pharmacy willingness of sales promotion with various incentives, and ensure stickness of the pharmacy patients.
3、Provide the patient-oriented one-stop medication value-added services, and obtain the effective patient medication feedback.
4、Create the private domain traffic pool, and help the enterprises to realize better online traffic sales transformation, offline traffic redirection and circular re-purchase.
1. Flexible product architecture
The Acctrue Technology coding system is applicable to various serialization coding production lines, and can seamlessly dock with multiple L4 system service providers.
2. Stable product performance
Acctrue Technology has developed in the industry of China's pharmaceutical electronic supervision code for over 10 years, serving more than 5000 customers and 15000 production lines.
3. Rich experience in overseas projects
Acctrue Technology established a branch in San Francisco of the United States in 2013. It can not only successfully provide the whole industry chain traceability service for the foreign pharmaceutical enterprises such as Pfizer, Bayer, AstraZeneca, Roche, Sanofi, Novartis, Astellas, CSL Behring, Octapharma and Grifols, but also provide the serialization construction for the domestic pharmaceutical enterprises to export products to the United States and the European Union such as Hengrui Medicine, Shijiazhuang Pharmaceutical Group, Techdow Pharmaceutical, Minsheng Pharmaceutical.
4. Excellent service capability
Acctrue Technology has more than 10 years of experience in pharmaceutical whole industry chain traceability, masters the consulting solutions of whole process, and occupies more than 85% of the pharmaceutical supervision market. It has gained rich implementation and service experiences in the field of pharmaceutical electronic supervision.
5. One-stop cloud service platform
LinkLink builds the one-stop cloud service platform for the pharmaceutical whole industry chain, and provides all-round and rapid solutions to the problems in terms of government regulation of pharmaceutical enterprises, patient traceability, anti-counterfeiting verification, compliance verification, and pharmaceutical supply chain and terminal empowerment.
